States of Matter
Solid, liquid and gas (Dimistry Volkov, iStockphoto)

Solid, liquid and gas (Dimistry Volkov, iStockphoto)
How does this align with my curriculum?
Learn about the states of matter, as well more about one state in particular - gas!
Chemistry helps us understand the properties and composition of the world around us. Here you will learn about the states of matter, as well as lots about one state in particular (three guesses – it’s not solid or liquid...).
The States of Matter
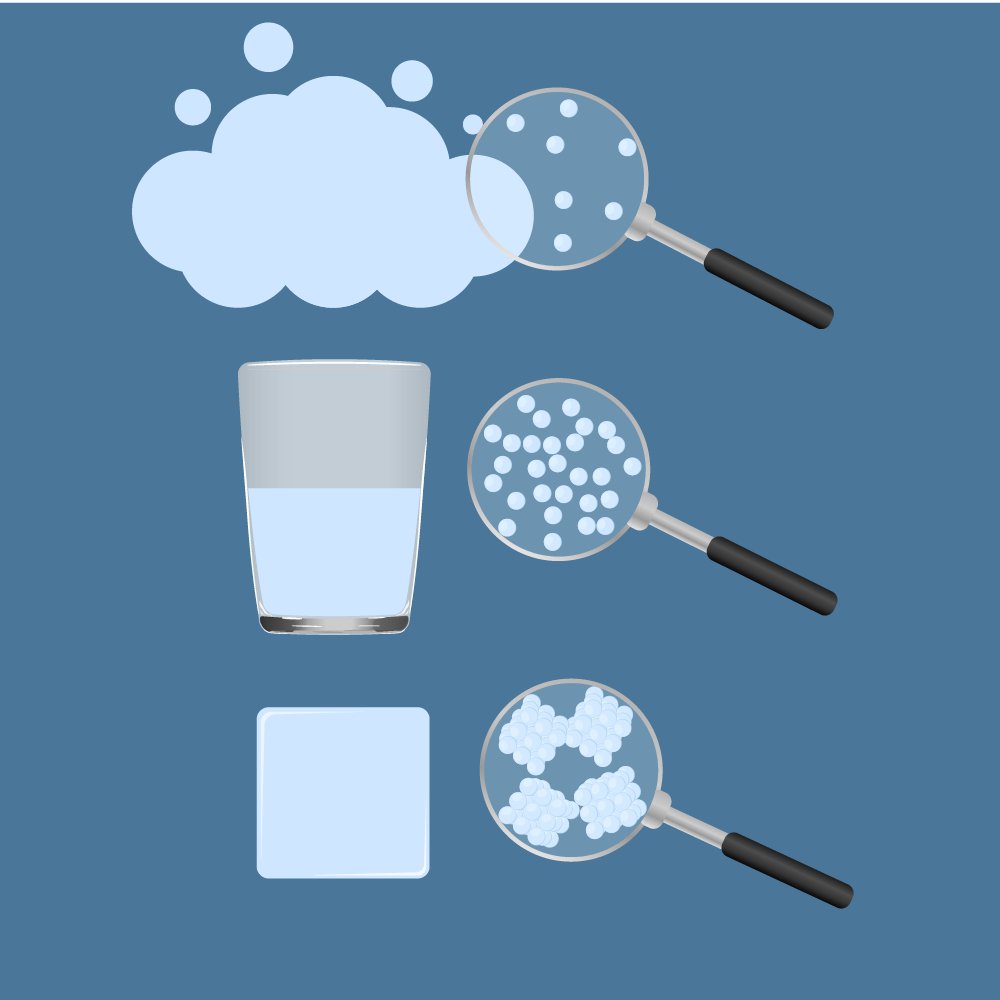
Depending on temperature, pressure and a substance’s properties, a substance can take on different physical forms. We call these physical forms States of Matter. There are three very well known states of matter: Solids, Liquids, and Gases. Other states of matter also exist. These include Plasma (a state of matter similar to a gas, but contains free-moving electrons and ions - atoms that have lost electrons) and Bose-Einstein Condensates (BECs) (waves of matter that can occur with some types of atoms at super cold temperatures).
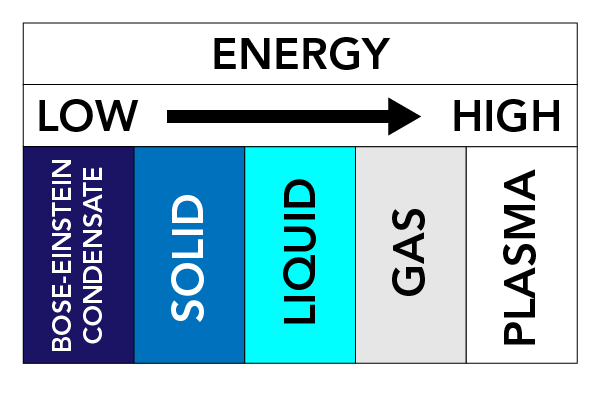
The forces between particles and the pressure on particles keep the particles together. If we warm up matter (add energy), the particles start moving faster and tend to spread apart. This movement of particles has a large effect on the state of a substance.
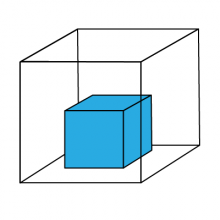
Solids
In solids, the forces keeping the particles together are relatively strong, and the particles stay very close to each other. The particles can vibrate but they are not moving around much. This is why solids are hard and rigid. Left on their own, solids will keep their shape.
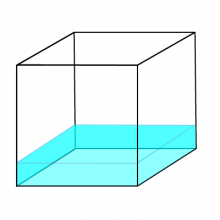
Liquids
In liquids, the forces between the particles are weaker than in solids. Particles are still fairly close together, but can move around freely. Liquids can flow around inside a container, and don’t have any particular fixed shape.
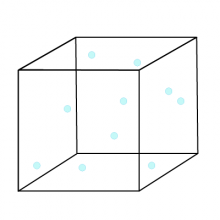
Gases
Gases are difficult to relate to because they are often invisible, but found all around. You can feel them when, for example, the wind blows. You can sometimes smell them when, for example, you smell the odour of food that is cooking, or when a skunk has been upset. Some gases are important for our health (e.g., oxygen) while others can be deadly (e.g., hydrogen sulfide and chlorine). Before surgery, you may receive an anaesthetic gas, which contains chemicals, to relieve pain and make you unconscious during the procedure. Gases are also responsible for the force of explosions. Let’s have a closer look at gases.
Types of Gases
There are elemental gases (made up of a single element) and gases that are compounds (made up of more than one element). The symbols of the elemental gases can be found in any Periodic Table of Elements. They are summarized in the chart below.
Diatomic Gas |
Chemical Formula |
Monatomic Gas |
Chemical Formula |
|
Hydrogen |
H2 |
Helium |
He |
|
Oxygen |
O2 |
Neon |
Ne |
|
Nitrogen |
N2 |
Argon |
Ar |
|
Fluorine |
F2 |
Krypton |
Kr |
|
Chlorine |
Cl2 |
Xenon |
Xe |
A diatomic gas is one in which the basic unit is a molecule made of two atoms joined together. A monatomic gas is one in which the basic unit is a single atom. Most gases, however, are compounds with two or more different elements chemically united. The most common one is water vapour, H2O. Here are the names, formulas and uses of some compound gases:
Common Name |
Chemical Formula |
Where you normally find it |
|
Carbon dioxide |
CO2 |
Atmosphere, car exhaust, pop, our lungs |
|
Propane |
C3H8 |
BBQs, camp stoves, fuel for some vehicles |
|
Methane |
CH4 |
Component of greenhouse gas and natural gas |
|
Ammonia |
NH3 |
Used to make fertilizers, cleaning products |
Learn More
Solid, liquid, gas and … plasma? - Michael Murillo (2015)
This video by TED-Ed explains how plasma is formed, and where it’s found in our universe.
Matter: Bose-Einstein Condensates
This article by Chem4Kids is an overview of the Bose-Einstein state of matter and how it’s created.
References
Bagley, M. (2019, August 21). Matter: Definition & the Five States of Matter. LiveScience.